Our Research
The Early Lung Cancer Action Project (ELCAP) for low-dose CT (LDCT) screening was initiated following several research retreats in 1991 and 1992 and then started in 1992 (1,2); these reference numbers in the article can be found here: https://pubmed.ncbi.nlm.nih.gov/32520848/
The ELCAP study employed an innovative prospective cohort design, making a direct comparison between LDCT and chest radiography (CXR), to evaluate the stage distribution of lung cancers detected by each method (9). Separate analyses are performed for the first round of screening, called the baseline round, and for subsequent rounds of repeated LDCT screening every 12 months, called annual repeat rounds (Figure 1). The ELCAP design also allows for random assignment of lung cancer patients to different treatment or watchful waiting options (3-10). For example, comparison of CT with another screening test (i.e., biomarkers) could use the same design.
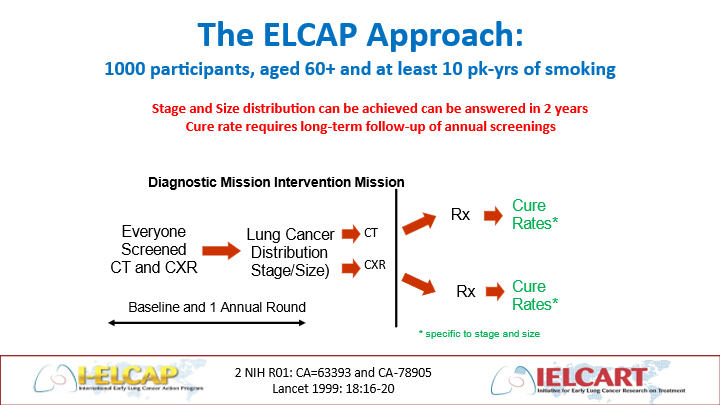
Figure 1. The ELCAP design allows for direct comparison of different screening tests (e.g., imaging tests or biomarkers) by providing the baseline round followed by as many repeat rounds of screening as needed to assess any particular comparison.
The baseline round showed that 85.1% of the LDCT-detected lung cancer patients had clinical stage I disease and CXR missed 82% of them (8). The annual repeat screenings were completed, submitted for publication in 1999, and published in 2001 (9, 10). These results gave renewed hope to people at risk of lung cancer throughout the world (11).
Starting in October 1999, the I-ELCAP Investigators have held the International Lung Cancer Screening Conferences every six months. Please click here to view our Conferences and Events page.
Since 2000, the ELCAP paradigm allowed for continued expansion of LDCT screening to New York State (NY)-ELCAP (17) and over 80 institutions in 10 countries (International (I)-ELCAP) (18), all using a common protocol (19). The common protocol and management system allowed for the emerging data to be pooled and analyzed in an efficient manner as widespread interest in screening soon became apparent (20-23). You can view the I-ELCAP Protocols by clicking here.
By 2006, the expanded I-ELCAP collaboration had sufficient follow-up of the participants diagnosed with lung cancer so that Kaplan-Meier survival analyses could be used to estimate cure rates of lung cancer when diagnosed under LDCT screening (1, 12-14).
In 2023, the 20-year Kaplan-Meier survival rates (https://pubs.rsna.org/doi/full/10.1148/radiol.231988) shown in Figure 2 confirmed the previously published 10-year rates. The estimated cure rate was 80% (95% CI: 74-85%) for all lung cancers diagnosed under screening (i.e., both screen- and interim-diagnosed). For patients in clinical Stage I and resected within 2 months of diagnosis, the rate was 92% (95% CI: 88-95%).
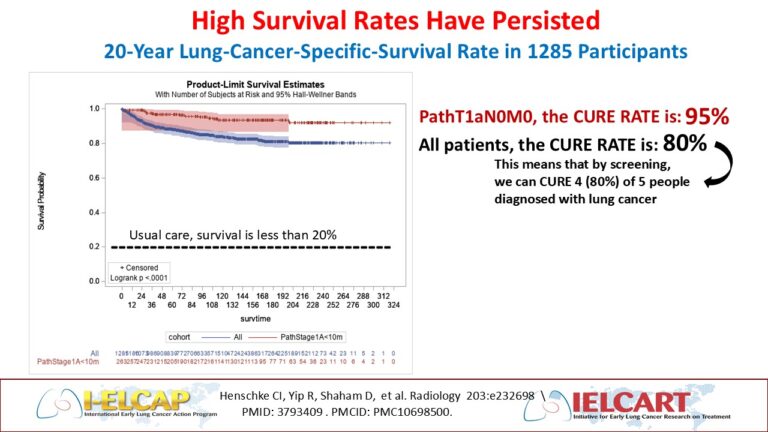
Figure 2. I-ELCAP Kaplan-Meier survival rates for all patients diagnosed with lung cancer under screening and for patients who had the earliest pathologic stage of pT1aN0M0.
The success of I-ELCAP is attributed to several key elements: its well-structured design that offered screening to individuals at high risk for lung cancer within a clinical program, the ELCAP Management System that ensured quality control and efficient management, and the use of an advanced registry which stored both radiologic images and related data.
Lessons learned over 25 years and publications are detailed in the following article (website to JTI article). Advances have been incorporated in the latest I-ELCAP protocol which also details the many valuable findings made on the screening CT along with the recommendations for follow-up and preventive health care.
I-ELCAP Investigators had started to use artificial intelligence (AI) in the 1990’s. *
Artificial Intelligence has advanced since this time, so we have started the IELCAP-AIRS project to fully integrate AI into the screening process: https://www.ielcap-airs.org/
I-ELCAP Publications
Check out our publications list here.
